RCA for Contamination in Pharmaceutical Batch
Contamination in pharmaceutical products can lead to significant risks, such as regulatory non-compliance, costly recalls, and compromised patient safety. Common causes include "contaminated raw materials," "dust or particle accumulation," and "ineffective cleaning processes." These issues often stem from gaps in Standard Operating Procedures (SOPs), poorly maintained equipment, or insufficient personnel training. ProSolvr, a Visual Gen AI-powered Root Cause Analysis application, equips pharmaceutical teams with the tools to pinpoint and eliminate contamination risks effectively.
ProSolvr enables teams to collaboratively map and resolve contamination issues in a user-friendly and intuitive environment. It simplifies the analysis of intricate systems, highlighting the interconnected causes of contamination. With clear visual representations, stakeholders can align on actionable solutions, ensuring that quality and safety standards are met consistently. The application’s focus on thorough and permanent problem resolution makes it an indispensable tool for addressing contamination challenges and safeguarding the integrity of pharmaceutical products.
For example, by visualizing the root causes, teams can address "non-compliance with packaging standards," "failure to follow aseptic techniques," or "equipment not properly cleaned." ProSolvr’s approach ensures every factor contributing to contamination is linked to actionable corrective steps, fostering confidence in the manufacturing process. With ProSolvr, pharmaceutical companies can systematically eliminate contamination risks, ensuring that products meet stringent safety and quality standards at every stage of production.
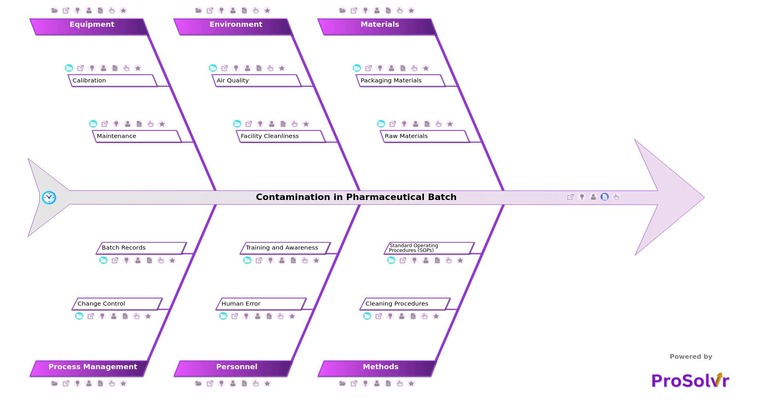
Fishbone diagrams, also known as Ishikawa diagrams, are essential tools for visualizing and identifying the root causes of Contamination in Pharmaceutical Batches. These diagrams break down the problem into clear categories such as Materials, Methods, Environment, Personnel, Equipment, and Process Management. Each category reveals specific contributors to contamination, like improper handling of raw materials, ineffective cleaning processes, and non-compliance with hygiene protocols. By mapping these potential causes, pharmaceutical teams gain clarity on the sources of contamination and can implement targeted corrective actions to maintain product safety, quality standards, and regulatory compliance. Utilizing a structured Fishbone diagram allows for a comprehensive understanding of contamination risks, enabling a proactive approach to resolving and preventing contamination issues in pharmaceutical production.
Who should use the Contamination in Pharmaceutical Batch template?
This Fishbone diagram template is ideal for teams seeking a comprehensive view of contamination sources in pharmaceutical products like:
- Quality Control Professionals: Ensures contamination sources are identified and managed during the production process.
- Pharmaceutical Engineers: Helps in pinpointing technical and procedural issues that may lead to contamination.
- Regulatory Compliance Officers: Assists in meeting regulatory standards by providing a clear view of contamination risks.
- Production Managers: Supports operational oversight by identifying process weaknesses that could contribute to contamination.
- Quality Assurance Teams: Facilitates thorough investigations into contamination incidents to maintain product integrity and safety.
Why use this template?
- Quality Assurance Insight: It provides invaluable insights for anyone focused on quality assurance and the safety of pharmaceutical products by highlighting common sources of contamination such as raw material handling, equipment calibration, and facility cleanliness.
- Simplicity: The Ishikawa diagram’s clear layout makes it user-friendly, allowing for an organized arrangement of causes and sub-causes. This structure facilitates an in-depth analysis of contamination issues, making it easy for teams to identify critical areas for improvement.
- Versatility: While designed for contamination issues in pharmaceuticals, this template can be adapted to analyze root causes across other industries where product purity, safety, or regulatory compliance is a concern, making it a versatile tool for various quality management applications.
Draft and create a template for problem analysis in ProSolvr by smartQED.